The genetic frontier: promises and perils in the age of human redesign
by Kai Ochsen
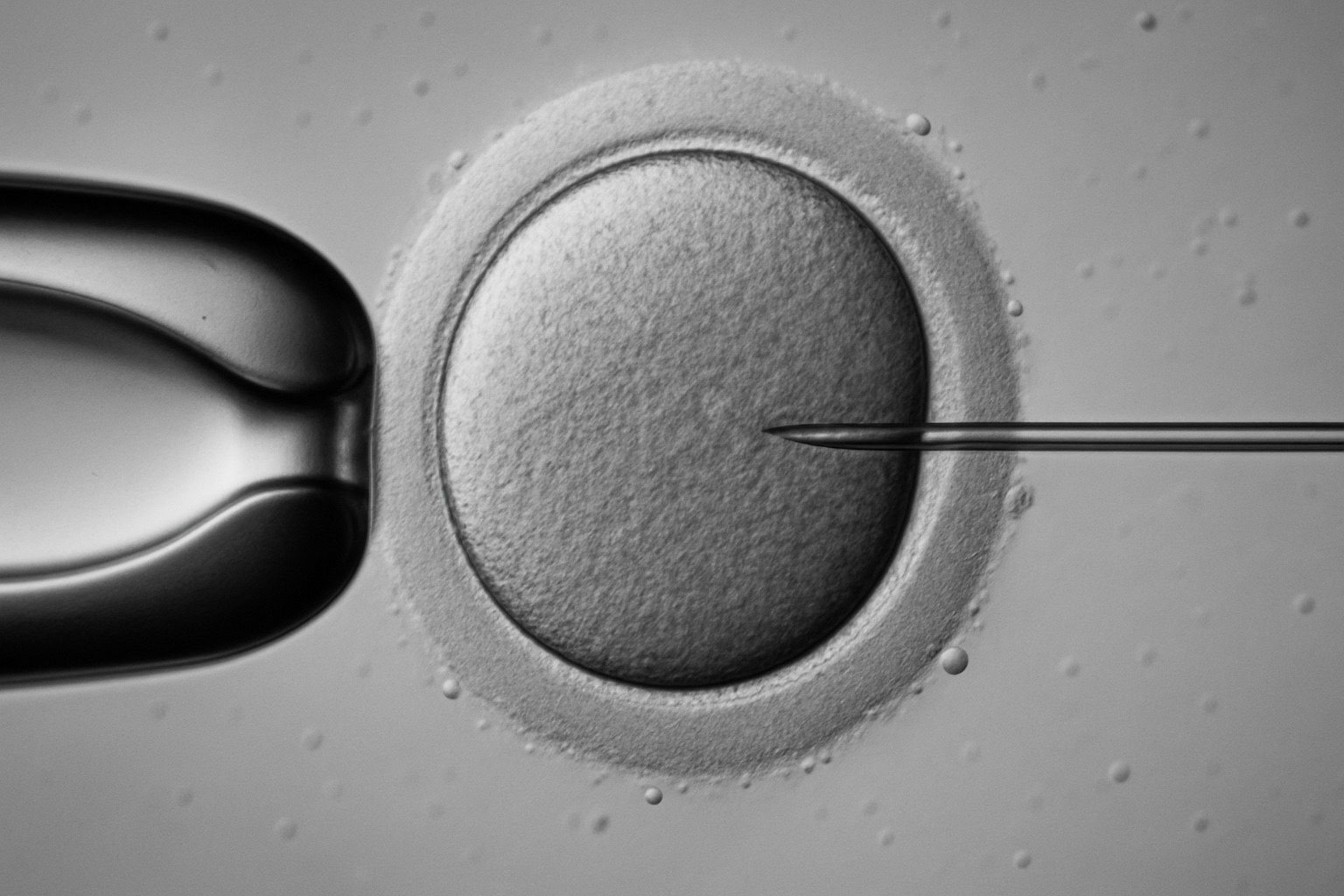
Science has long flirted with the idea of remaking humanity, yet only in the past decade has that notion crossed from speculative fiction into tangible laboratories. The latest breakthrough, reported by researchers at Oregon Health & Science University, shows the creation of human eggs from skin cells, fertilized successfully, though riddled with chromosomal errors, and ultimately halted before any pregnancy attempt. The experiment, cautiously described as a proof of concept, represents both an extraordinary leap forward and a reminder of the immense biological and ethical terrain still ahead.
This achievement did not happen in isolation. It sits atop the growing edifice of gene-editing tools like CRISPR, which have already demonstrated their potential to correct mutations, erase hereditary conditions, and prevent certain diseases before birth. Together, these technologies invite us to imagine futures where the boundaries of biology are rewritten, where infertility is overcome not by donation but by fabrication, where genetic disorders like cystic fibrosis or Huntington’s disease are removed from the human lineage, and where the notion of natural limits begins to erode under the weight of human ingenuity.
But every promise carries its shadow. To conceive healthier, longer-lived, less vulnerable humans is also to confront the specter of design: who decides which traits are desirable, which lives are worth correcting, and which conditions merit elimination? The elimination of conditions such as Down syndrome, already framed by some as progress toward equality, has also sparked deep debates about dignity, identity, and the risk of reinforcing the very discrimination society claims to erase.
Equally complex are the social questions: what does it mean if only the wealthy can afford enhanced fertility or genetic editing? Could late pregnancies, once fraught with danger, become routine, while poorer families face an expanding gulf of health inequality? The technology, though framed as a cure for suffering, also threatens to reproduce, even magnify, the same patterns of exclusion it seeks to overcome.
At its core, this new genetic frontier forces us into an uncomfortable but necessary reflection: are we solving problems of nature, or merely transferring them into new moral and political dilemmas? The laboratory egg fertilized in Oregon may never develop into a human child, yet it symbolizes a threshold moment, the crossing of an invisible line between what humanity has received from biology and what it might soon manufacture.
This essay will follow that trajectory. We will explore the mechanisms of CRISPR and lab-made gametes, the medical hopes tied to erasing hereditary suffering, and the risks of new forms of inequality. We will examine how fertility, disability, and identity intersect in this genetic revolution, and why regulation and ethics are lagging behind the pace of science. Finally, we will consider how these developments tie into the wider vision of Transhumanism, the philosophical movement that seeks to transcend human limitations, and whether that path represents salvation, hubris, or both.
CRISPR and the genetic frontiers
When CRISPR-Cas9 first entered the scientific stage, it was presented as a scalpel for the genome. A tool elegant in its simplicity, it allowed researchers to cut DNA at precise locations and, in theory, to replace faulty segments with healthy sequences. What was once a dream of science fiction, the idea of editing the genetic code as if it were text, suddenly became a matter of laboratory practice. Within a few years, experiments in plants, animals, and human cells revealed both its extraordinary potential and the complexity of applying it safely.
The story of CRISPR’s rise is often told as a triumph of serendipity. Borrowed from a bacterial defense mechanism against viruses, it became a universal instrument for biology. With it, scientists can deactivate genes responsible for certain diseases, insert beneficial traits, or even engineer organisms resistant to pathogens. Beyond medicine, CRISPR has transformed agriculture, enabling crops that withstand drought or resist infection. Yet in the medical realm, the stakes are higher, here, editing is not about higher yields but about rewriting the biological destiny of future generations.
In human health, CRISPR trials are still in their infancy, but the results have been remarkable enough to capture public imagination. In some cases, experimental therapies have restored sight to those with inherited blindness, or have offered new pathways for sickle cell anemia. The notion that genetic diseases could be corrected at their root, not just managed, represents a conceptual revolution in medicine. It suggests that suffering can be prevented before it begins, that a lineage carrying a cruel mutation might finally be freed from its burden.
But the very ease of CRISPR provokes anxiety. Unlike earlier genetic engineering methods, this system is cheap, accessible, and relatively simple to use. Small laboratories, even hobbyists, can experiment with gene editing. That democratization is double-edged, it accelerates research but also raises the specter of misuse. The infamous case of He Jiankui, who in 2018 announced the birth of gene-edited twins, revealed how quickly the line between curiosity and recklessness can be crossed. His attempt, condemned worldwide, was framed as protecting the children from HIV, yet it was also a premature, ethically unvetted step into altering the human germline.
CRISPR’s promise has therefore collided with regulatory caution. In many countries, germline editing is either banned or strictly controlled, reflecting deep societal unease about tampering with the inheritance of humanity. This is not merely a matter of technical safety, though off-target effects remain a serious concern. It is also about values, whether human beings should claim authority over the code that made them, and what it would mean to wield that authority at scale.
Some argue that the fear is overstated. They point out that medicine has always interfered with nature: vaccines, antibiotics, organ transplants, all once seemed radical. By that logic, CRISPR is just another stage in the progression of human care. Yet the counterpoint is strong, unlike other interventions, editing the germline is irreversible, passing not only to one patient but to all their descendants. This amplifies the responsibility beyond individual choice, extending it into the future of the species.
The genetic frontier therefore appears less like a smooth path and more like a crossroads. On one side lies the potential to eliminate entire categories of disease, to free millions from inherited suffering, and to extend the possibilities of life itself. On the other side lies the risk of errors, inequality, and a slide into genetic stratification. For every parent who dreams of sparing their child from a cruel condition, there is another who fears that such power will divide humanity into classes of the modified and the unmodified.
The Oregon experiment with lab-made eggs must be seen in this wider context. It is not simply an isolated technical feat, but a continuation of the CRISPR narrative, the sense that biology is becoming programmable. As the boundaries blur between natural reproduction and engineered design, society will face questions not only of safety but of meaning. To edit the genome, or to create eggs from skin cells, is not only to solve a medical problem, it is to redefine what it means to inherit, to be human, and perhaps, to belong.
Genetic diseases: between treatment and prevention
For most of human history, hereditary diseases were endured rather than treated. Families watched as conditions like hemophilia, muscular dystrophy, or Huntington’s disease struck generation after generation, each case both a personal tragedy and a reminder of the inescapable weight of inheritance. Medicine could offer only limited relief, symptom management, supportive care, sometimes experimental drugs that slowed progression but rarely cured. The underlying problem, a mistake coded into DNA, remained untouchable.
The rise of modern genetics reframed that struggle. Once researchers learned to identify mutations, they could trace the molecular origins of disease. Diagnosis became more precise, enabling families to know what they carried and what risks their children might face. Genetic counseling emerged as a discipline, offering guidance but also introducing new forms of anxiety: knowledge without cure, foresight without remedy. For some, this led to difficult reproductive choices, from selective IVF to the decision not to have children. The information, however powerful, remained powerless to change the code itself.
CRISPR and related technologies altered this horizon. For the first time, the idea of intervening at the level of DNA suggested not just treatment but prevention. Instead of preparing for a disease to unfold, science could in principle delete it from existence. Correcting a mutation in an embryo or germline cell could break the chain of inheritance altogether, turning what was once inevitable into avoidable. The symbolic weight of this possibility is immense, it transforms disease from destiny into an option.
The Oregon experiment with lab-made eggs adds another layer to this transformation. If gametes themselves can be manufactured, the scope of who can reproduce expands dramatically. Same-sex couples, people with infertility, or those with depleted egg reserves could, in theory, create biological children with fewer limitations. Yet the deeper implication is that these eggs could also be edited before fertilization, combining synthetic reproduction with genetic correction. This convergence of technologies, artificial gametogenesis and gene editing, pushes the boundary further away from treatment and closer toward the engineering of outcomes.
Critics warn that this transition carries profound risks. The notion of prevention easily slips into selective elimination. Down syndrome provides a vivid example, in countries where prenatal testing is widespread, termination rates are high, reducing the number of children born with the condition. Disability advocates argue that this trend reflects not progress but prejudice, that the drive to eliminate a genetic difference can devalue those who live with it. What one group sees as compassion, another sees as erasure.
This tension reveals a crucial dilemma: when does medicine’s duty to relieve suffering become society’s attempt to define which lives are worth living? If prevention means wiping out entire categories of human variation, we must ask whether we are curing disease or curating humanity. Such questions are not hypothetical, they are already playing out in prenatal clinics, IVF centers, and legislative debates across the globe.
Yet to dismiss prevention entirely would also be unjust. Families devastated by lethal mutations often see gene editing not as eugenics but as liberation. To imagine a future where a child is spared the agonies of Tay-Sachs or cystic fibrosis is not sinister, it is profoundly humane. The challenge lies not in rejecting the technology outright but in guiding its use with principles that respect both individual dignity and collective diversity.
At this intersection of treatment and prevention, we glimpse the double-edged nature of the genetic frontier. It offers unprecedented hope but also invites us to confront uncomfortable truths about value, difference, and identity. The debate will not be resolved by science alone, it requires an ongoing negotiation between what technology makes possible and what society chooses to accept. And as the pace of discovery accelerates, those choices may come faster than we are prepared to make them.
Late pregnancies and fertility extensions
The biological clock has always been one of nature’s strictest enforcers. For women, fertility begins to decline sharply after the mid-thirties, and by the age of forty, the chances of conception drop significantly while risks of miscarriage and chromosomal abnormalities rise. This biological timeline has dictated life choices for centuries, when to marry, when to bear children, how many children to have. Even as society modernized, and women increasingly delayed childbearing to pursue education and careers, the limits of biology remained largely unmoved.
Advances in reproductive medicine began to loosen those constraints. In vitro fertilization, egg freezing, and hormonal treatments created new opportunities, but these were extensions rather than solutions. They could buy time, but not reverse the age-related decline in egg quality. As women lived longer, healthier lives and sought children later, the biological barrier persisted. It is here that the idea of lab-made eggs intersects powerfully with social reality, if functional eggs can be created from skin cells, the tyranny of the biological clock may one day be lifted.
For women who wish to conceive in their forties or even fifties, such technology could be revolutionary. No longer would fertility be tethered to youth, the choice to delay parenthood would become less fraught with risk. The implications ripple outward, family structures could shift, career planning might evolve, and the narrative of too late could fade. In a world where late-term pregnancies are safe and viable, society would need to reimagine not only medicine but also the cultural expectations placed upon women.
Yet extending fertility raises questions that transcend biology. Should medicine normalize childbirth at ages once considered dangerous? What does it mean for children to be born to parents of advanced age, not as exceptions but as part of a broader social trend? Advocates argue that healthier reproduction at older ages would reduce stigma and increase freedom of choice. Critics caution that it could also lead to generational imbalances, where parents are already elderly when their children reach adulthood, potentially burdening both sides of the family equation.
The ethical landscape is equally complex. If lab-made eggs become safe and reliable, they could initially be available only to those who can afford them. This exclusivity would reinforce inequalities, granting reproductive freedom to the wealthy while leaving others bound to nature’s limits. Over time, the pressure might grow to make such procedures more widely accessible, but questions of cost, prioritization, and fairness would remain. Medicine’s history suggests that new technologies often amplify existing disparities before they democratize.
There is also the matter of identity and tradition. Reproduction has long been a deeply personal, often spiritual act, shaped not only by biology but by cultural and religious values. The idea of creating eggs in a laboratory, detached from the natural process of ovulation, may unsettle communities that view fertility as sacred. For some, this will symbolize liberation, for others, it will feel like transgression. The clash between technological possibility and cultural meaning will likely be as contentious as the scientific debates themselves.
Still, the transformative potential cannot be denied. For women facing infertility due to age or medical conditions, lab-made eggs represent a path toward motherhood that once seemed impossible. They embody a redefinition of what it means to have a biological child and challenge the fatalism that has accompanied reproductive decline. In this sense, the Oregon experiment is not just a technical achievement but a hint of a social revolution waiting on the horizon.
Late pregnancies and fertility extensions thus highlight the duality of the genetic frontier, freedom intertwined with uncertainty, empowerment shadowed by risk. To lift the limits of biology is to grant choice, but it also compels us to confront questions of justice, culture, and intergenerational balance. As science moves forward, society will be asked not just whether it can accept this transformation, but whether it is prepared to live with its consequences.
The promise of healthier, less vulnerable humans
The dream of medicine has always been to reduce suffering, to shield humanity from the cruelty of disease. Vaccines eradicated smallpox, antibiotics turned once-fatal infections into inconveniences, and surgical innovations restored lives thought lost. Yet none of these advances altered the fundamental randomness of genetics. Some individuals are born more fragile, carrying predispositions to cancer, heart disease, or rare syndromes that no lifestyle change can fully prevent. To imagine a world where these vulnerabilities can be corrected before birth is to contemplate a new chapter in the story of human resilience.
The appeal is undeniable. If CRISPR and artificial gametogenesis could erase mutations responsible for cystic fibrosis, sickle cell anemia, or Duchenne muscular dystrophy, millions of families would be spared the agony of watching loved ones deteriorate. Preventing suffering at its root, rather than treating it downstream, is both efficient and compassionate. It promises not just healthier individuals, but healthier societies, where resources currently consumed by chronic care could be redirected to education, opportunity, or innovation.
Beyond eliminating specific diseases, the prospect extends to reducing predispositions. What if genetic markers for Alzheimer’s or certain cancers could be corrected? What if cardiovascular risks could be minimized before a child even took its first breath? Such interventions would not guarantee immortality, but they could dramatically alter life expectancy and quality of life. Human beings might grow not only older, but stronger, spending more years in good health, free from vulnerabilities that once seemed inevitable.
The social implications are profound. A healthier population is, in theory, a less discriminated one. Much of social stigma revolves around perceived weakness: disability, illness, or conditions that mark individuals as different. If these differences could be reduced or eliminated, advocates argue, society might become more inclusive by default. Prejudice rooted in biology could lose its grip. The argument is seductive, the idea that genetic correction is not only a medical act but a social equalizer, reshaping the very conditions of discrimination.
But such optimism carries hidden assumptions. It presumes that discrimination is solely tied to biology, when in fact it is deeply cultural. History shows that societies have always found new ways to marginalize others, whether through race, class, religion, or ideology. To imagine that curing disease alone will eliminate prejudice risks underestimating the adaptability of exclusion. A world without Down syndrome, for example, might not be more compassionate, it might simply direct its intolerance elsewhere.
Furthermore, the promise of healthier humans raises the question of what counts as health. If vulnerability is defined too broadly, the push for genetic perfection risks sliding into enhancement: not just correcting disease but optimizing traits, from intelligence to athletic ability. The line between healing and upgrading becomes blurred, and with it, the definition of fairness. Would a genetically optimized child have an unfair advantage over one born naturally? Would the unedited be seen as irresponsible, or even inferior?
Despite these dangers, the vision continues to captivate. The ability to reduce suffering at its root remains one of the most compelling moral arguments in favor of genetic technologies. It speaks to a universal human desire, not only to live, but to live well, free from the burdens of inherited fragility. If carefully governed, such advances could indeed mark the beginning of a more robust human species, less vulnerable to the blind lottery of genetics.
The promise of healthier humans thus embodies both hope and paradox. It invites us to imagine a society where disease no longer defines destiny, yet it also forces us to recognize that vulnerability is not only biological but social, cultural, and ethical. To cure one form of fragility may leave another untouched, or even create new ones. In seeking to master biology, humanity must also confront the deeper question of whether vulnerability is an enemy to be eradicated, or an essential part of what makes us human.
The specter of eugenics and inequality
The word eugenics is one of the most haunting in the history of science. It conjures images of forced sterilizations, state programs of racial purification, and the pseudo-scientific justifications for some of the darkest atrocities of the twentieth century. Today’s genetic technologies are far removed in method and intention, yet the resonance of that history lingers. Whenever discussions arise about eliminating diseases or enhancing traits, the specter of eugenics inevitably shadows the conversation.
This connection is not merely rhetorical. The original eugenics movement framed itself as a form of progress, the improvement of humanity through careful breeding and the removal of undesirable traits. The language was couched in terms of health and societal benefit, not cruelty. Yet beneath the veneer of science lay prejudice, coercion, and the imposition of narrow definitions of value. The danger today is not that scientists will resurrect eugenics deliberately, but that society might drift into its logic unconsciously, through the cumulative effect of individual choices made within systems of inequality.
Consider the possibility of genetic editing becoming widespread but not universally accessible. If only wealthy families can afford to correct mutations or enhance traits, disparities would deepen in ways both visible and invisible. Healthier, stronger, and potentially more cognitively advantaged children could be born to the privileged, while others would remain vulnerable to the genetic lottery. Over generations, this would create a stratification not just of wealth, but of biology, a division between those who are edited and those who are not. The result would be a form of genetic classism, one that echoes the logic of eugenics without requiring state intervention.
Even when framed as prevention of suffering, certain practices can carry eugenic undertones. The reduction of Down syndrome births in countries with extensive prenatal screening is one such example. While framed as a matter of parental choice, the near-erasure of a community raises difficult questions about what society values. Disability advocates warn that such trends send a chilling message, that lives with certain conditions are less worth living. What begins as medical prevention can quickly resemble social elimination.
The rhetoric of better is especially treacherous. When scientists or policymakers speak of producing healthier humans, what definitions underlie that word? Who decides which traits count as disease, and which are simply variation? Homosexuality, once pathologized, is now understood as a natural expression of human diversity. What traits today might be considered disorders, but tomorrow recognized as difference? The line between treatment and normalization is not fixed, and the power to define it carries immense political and moral weight.
Moreover, the specter of eugenics is tied not only to the elimination of difference but also to the pursuit of perfection. If technology can correct vulnerabilities, the temptation will be strong to go further, to enhance intelligence, physical prowess, or aesthetic traits. The language of choice masks the pressure that cultural norms and economic competition can exert. Parents may feel compelled to edit, not because they desire perfection, but because they fear disadvantaging their children in a world where others have already embraced the technology. In this way, enhancement can become less about freedom and more about conformity.
The issue is not hypothetical. We already see early forms of stratification in reproductive technologies. IVF, surrogacy, and genetic screening are more accessible to some than others, creating differences in who can control the conditions of reproduction. The leap from these disparities to genetic editing is not as wide as it may appear. What is at stake is whether society will allow technology to magnify inequality, or whether it will take steps to ensure that its benefits are shared.
Ultimately, the fear of eugenics reminds us that the pursuit of a better humanity is never just a scientific project, it is a social one. It reflects the values, prejudices, and structures of the societies that wield the tools. Science may provide the mechanism, but society determines the purpose. Without vigilance, the noble desire to heal can slide into the dangerous drive to perfect. The specter of eugenics lingers not because we intend to repeat history, but because history shows how easily ideals of progress can be twisted into exclusion.
Ethical and regulatory roadblocks
Scientific discovery often outpaces the frameworks of oversight designed to contain it. The development of CRISPR, artificial gametogenesis, and lab-made embryos has advanced so quickly that laws and ethical guidelines are struggling to keep pace. For every experiment that pushes the boundary of possibility, a parallel debate emerges about whether it should have been attempted at all. In genetics, this tension is especially stark, because consequences extend not only to patients but to future generations.
In many countries, germline editing is either prohibited or heavily restricted. Legislators and ethics boards worry about unintended outcomes: errors that could ripple through family lines, or societal shifts that could widen inequality. The United States, for example, still blocks federal funding for embryo-level genetic manipulation. Europe enforces strict limits, while other nations move faster, creating the risk of genetic tourism, where families seek procedures abroad that are banned at home.
The uneven pace of global regulation underscores the borderless nature of science. A breakthrough in one country reverberates worldwide, whether or not others are ready to follow. The case of the gene-edited twins in China demonstrated how a single act, taken outside formal consensus, can ignite an international firestorm. It also revealed the fragility of attempts to establish a shared global framework, showing how easily national priorities can fracture collective caution.
Ethics committees, often dismissed as bureaucratic obstacles, are among the few safeguards against reckless progress. They remind us that innovation without reflection can cause unintended harm. Yet ethics themselves are not universal. Cultural and religious traditions shape how societies interpret reproduction, family, and the sanctity of life. What one country views as unacceptable, another may frame as compassionate use of technology. This absence of a common baseline makes regulation a patchwork prone to loopholes.
The line between research and application is another fault line. Scientists argue that proof-of-concept experiments, such as fertilizing lab-made eggs, are essential to build knowledge. Critics counter that even symbolic steps normalize the idea of designing reproduction. Once feasibility is demonstrated, pressure often grows to translate it into practice, regardless of whether safety is fully established. The slope from theory to clinical application is not hypothetical, it is part of science’s natural momentum.
Regulation is not just about restraint, it also shapes the direction of progress. If laws are too restrictive, innovation may stagnate, or shift underground where oversight disappears. If they are too permissive, the market may drive the technology toward profit rather than equity. The tension between freedom to innovate and the duty to protect defines one of the most difficult policy challenges of our time.
Ethical and regulatory roadblocks, then, are not merely barriers to be cleared. They are mirrors held up to society, forcing us to ask what kind of progress we want and at what cost. To rush ahead without caution is dangerous, but to freeze in fear risks losing opportunities to relieve suffering. The challenge is not to halt the march of genetics, but to guide it with values as well as ambition.
Future visions: humanity in the age of genetic choice
The horizon of genetic technology invites us to imagine futures that are both inspiring and unsettling. If reproduction can be decoupled from biology, if embryos can be designed to eliminate disease, and if traits can be chosen rather than received, then humanity enters an era of genetic choice. This is not simply a matter of curing illness, but of redefining how people are born and what it means to inherit.
One vision is optimistic: a world in which genetic suffering is minimized, where parents no longer fear passing on cruel mutations, and where medicine has shifted from treatment to true prevention. Children could be born with stronger resistance to disease, healthier lifespans, and reduced vulnerabilities. Society, relieved of much of the burden of chronic illness, could invest more deeply in education, creativity, and social development. In this view, genetics is a tool of liberation, enabling people to flourish beyond the constraints of nature.
Yet the other vision is darker. If genetics becomes a marketplace, then choice itself could fracture society. Wealthy families could afford advanced editing, creating children who are not only healthier but enhanced in subtle ways, faster, sharper, or more resilient. Those without access would remain vulnerable to hereditary risks, widening the gulf between the genetically privileged and the genetically disadvantaged. The dream of equality through medicine could instead harden into a new hierarchy.
The language of choice complicates this further. It suggests freedom, yet choices are never made in isolation. Social norms and cultural pressures shape what parents consider acceptable. If a society begins to expect “responsible” parents to edit out certain traits, the freedom not to edit may become stigmatized. In such a climate, choice becomes a disguised form of coercion, where refusing intervention is judged as negligence. What appears voluntary may be anything but.
Future visions must also reckon with identity. If human traits are selected or engineered, how will individuals perceive themselves? A child born from edited embryos may wonder whether their achievements are their own or the product of design. Similarly, communities historically defined by certain genetic conditions may dwindle or vanish, raising questions of cultural continuity. To erase a disease may also mean erasing a shared identity, however painful that identity may be.
The politics of genetics will be just as influential as the science. Nations may compete to offer the most advanced interventions, seeing them as markers of technological superiority. Military research may consider editing for resilience or endurance. Companies will see opportunities for profit, shaping what is offered and at what price. In this race, the collective good may be overshadowed by strategic interests, turning genetics into another domain of global rivalry.
At the same time, some see an opportunity for a new social contract. If the benefits of genetic technologies are distributed equitably, they could reduce suffering on a scale no previous medical innovation has achieved. By integrating ethical safeguards, ensuring fair access, and maintaining transparency, humanity could enter a future where technology truly serves the many rather than the few. This requires foresight, cooperation, and above all, a commitment to justice that goes beyond national or corporate agendas.
Still, the future is unlikely to resolve neatly into utopia or dystopia. It will almost certainly be a mixture of both, where extraordinary gains coexist with new dilemmas. Some diseases may vanish, while new forms of inequality emerge. Some lives may be enriched, while others are complicated by questions of authenticity or belonging. The complexity of human society ensures that no genetic revolution will deliver only benefits without costs.
Ultimately, the visions of genetic choice reflect not only what science can achieve but what humanity chooses to prioritize. Will we embrace genetics as a shared resource, or allow it to deepen divides? Will we value diversity, or seek uniformity under the banner of progress? The answers will shape not only medicine, but the very meaning of what it is to be human in the centuries ahead.
Transhumanism and the redesign of humanity
The conversation about genetics ultimately leads into a larger philosophical movement: Transhumanism. At its core, transhumanism envisions the use of technology to transcend human limitations, biological, cognitive, even existential. It is the belief that humanity can and should take control of its own evolution, moving beyond the constraints of nature to craft a new, enhanced species. For some, this represents the next step in progress; for others, it feels like a betrayal of the very essence of being human.
Genetic technologies fit seamlessly into this vision. The ability to engineer embryos, suppress disease, and extend fertility aligns with the transhumanist goal of self-directed evolution. If humans can choose not only how to live but how to be born, the line between natural inheritance and conscious design disappears. What once belonged to chance becomes subject to deliberate choice, making biology a canvas rather than a given.
Supporters of transhumanism often frame it as an extension of medicine’s oldest mission: to relieve suffering and prolong life. From this perspective, eliminating hereditary diseases or expanding human capabilities is no different than introducing vaccines or developing antibiotics. Each innovation moves us further from the vulnerability of nature and closer to mastery over it. The dream is of a world where illness, disability, and even aging are no longer inevitabilities but obstacles to be engineered away.
Yet critics argue that the transhumanist project carries hidden dangers. To pursue perfection risks reducing humanity to a set of optimized traits, stripping away the very imperfections that give life meaning. The desire to eliminate vulnerability may erode compassion, if suffering disappears from our experience, will empathy fade as well? What happens to the bonds of community when human life is defined by design rather than by shared fragility? These are not merely abstract questions; they cut to the heart of what it means to belong in a human world.
The ethical dimension of transhumanism is inseparable from its social consequences. If enhancements become real, will they be shared equitably, or will they create new forms of biological inequality? A society where some can afford to edit their children into healthier, more capable versions, while others cannot, risks splitting into classes defined not by opportunity but by genetics. The pursuit of transcendence may leave behind those who cannot pay the price of admission.
There is also a spiritual undercurrent to these debates. Many traditions hold that human life is sacred precisely because it is not self-authored. To take charge of our own design is, in this view, an act of hubris, a claim to power that belongs to nature, fate, or the divine. Others counter that human creativity itself is sacred, that to invent and improve is not defiance but fulfillment of our potential. Between these poles lies a vast space of uncertainty, where the meaning of human dignity is contested.
For individuals, the prospect of genetic choice raises intimate questions of identity. Would a child engineered for certain traits feel gratitude, or burdened by expectation? Would they see themselves as free, or as products of parental design? The psychology of being “made” rather than “born” has not yet been tested, but it could shape generations to come. Identity, already fragile in a world of constant social comparison, may become even more complex when rooted in deliberate genetic decisions.
Transhumanism also reframes mortality. If science can reduce disease and extend lifespan, then aging itself becomes the final frontier. The dream of radical longevity is alluring: more years to learn, to create, to love. But it also raises questions about meaning. If life stretches indefinitely, does it lose urgency? Would a world of near-immortals stagnate, locked in cycles of repetition, or would it flourish under the wisdom of centuries-old minds? The answers remain speculative, but they underscore how deeply genetics ties into the human search for purpose.
What makes this debate urgent is that the future is not distant. The Oregon experiment with lab-made eggs, the growing success of CRISPR therapies, and the momentum of biotechnological investment all suggest that the first steps toward a transhumanist future are already being taken. The question is no longer whether humanity can alter itself, but whether it should, and how far it should go. The boundary between treatment and enhancement, compassion and coercion, liberation and inequality will not be drawn by science alone, but by collective choices.
Personally, I believe the value of genetics lies less in the pursuit of perfection than in the possibility of freedom from unnecessary suffering. To prevent children from inheriting cruel diseases, to extend reproductive opportunities, to reduce vulnerabilities that shorten life, these are humane and defensible goals. But the temptation to go further, to design traits for advantage, to create hierarchies of the edited and unedited, must be resisted with equal strength. The dignity of humanity lies not in perfection, but in the balance between what we can control and what we must accept.
In the end, transhumanism forces us to confront the most profound question of all: what does it mean to be human when humanity is no longer given, but made? The laboratory egg fertilized in Oregon is not a child, yet it is a symbol of the future that awaits. Whether that future becomes a story of liberation or of loss will depend not only on science, but on our willingness to guide it with humility, compassion, and a recognition that to be human is to be more than the sum of our genes.